Titration of CH3COONa with HCl and pKa determination from half equivalence point - Chemistry Stack Exchange

SOLVED: A buffer is prepared using acetic acid, CH3COOH, (a weak acid, pKa = 4.75) and sodium acetate, CH3COONa (which provides acetate ions, the conjugate base), according to the following proportions: Volume

Aqueous solution of HNO3,KOH,CH3COOH and CH3COONa of identical concentrations are provided. The pair(s) of solutions which form a buffer upon mixing is (are):

In a mixture of acetic acid and sodium acetate, the ratio of concentrations of the salt to the acid is increased ten times. Then the pH of the solution:
Why does the solution of sodium acetate give more concentration of Hydroxide ion? Shouldn't the number of Hydroxide ion and hydrogen ion be equal? - Quora


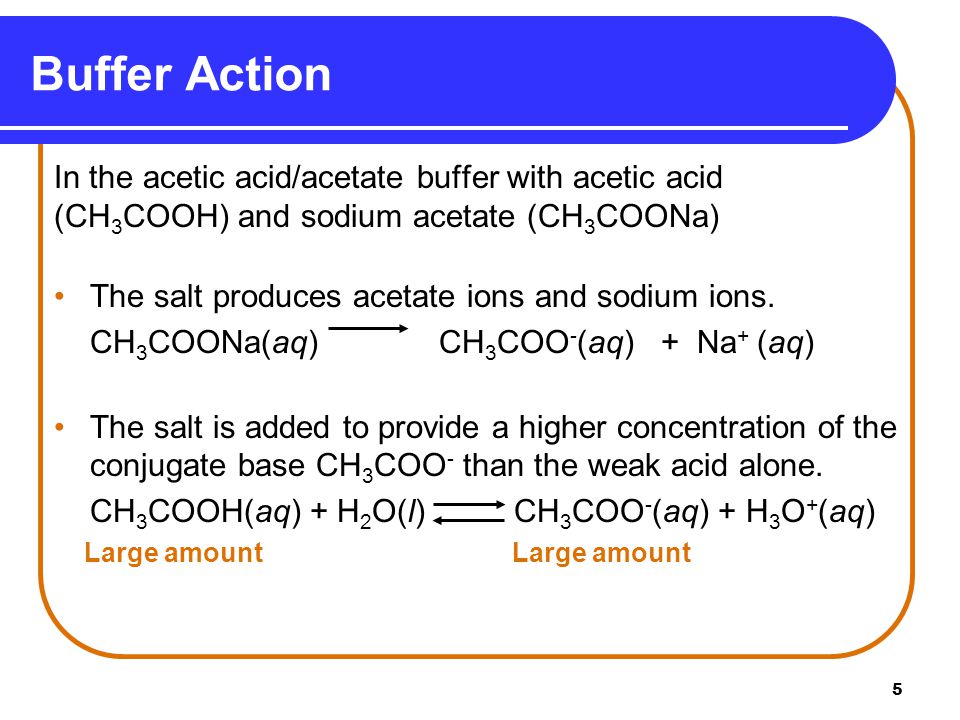



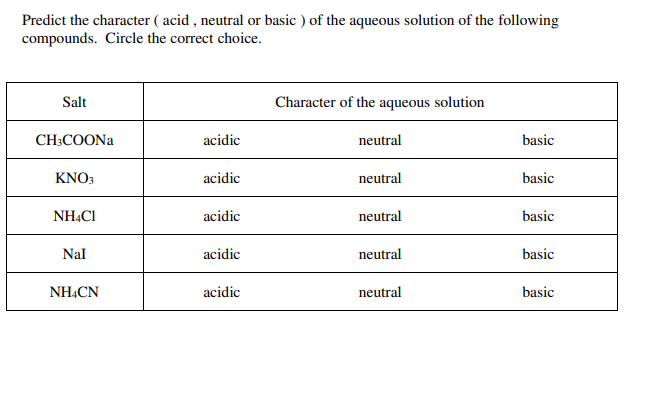






![Solved [10]14. Calculate the pH of a 0.25 M solution of | Chegg.com Solved [10]14. Calculate the pH of a 0.25 M solution of | Chegg.com](https://media.cheggcdn.com/study/693/693bb348-dd36-495c-8df3-30ac5efad5a9/image.png)