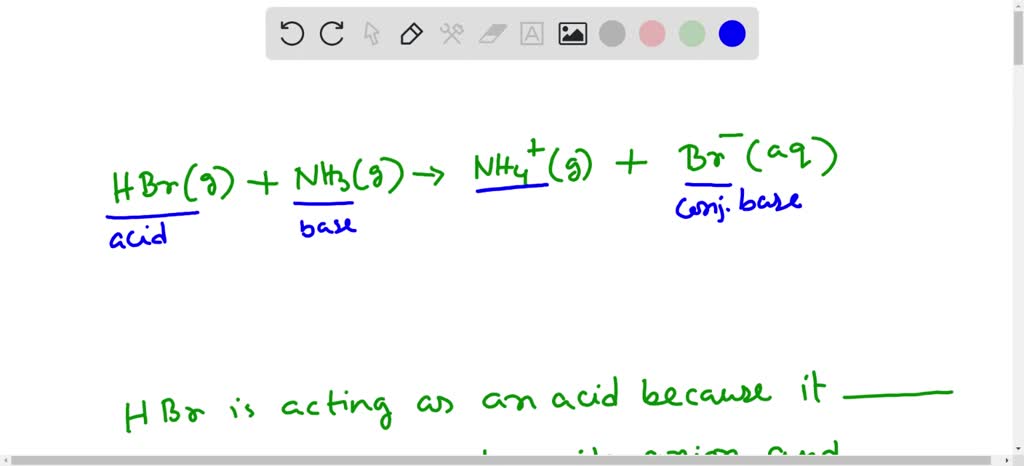
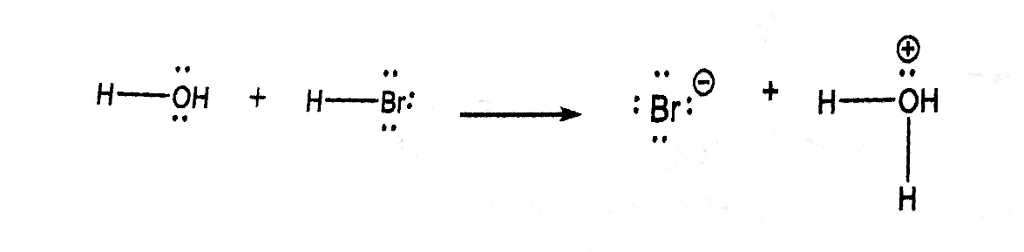
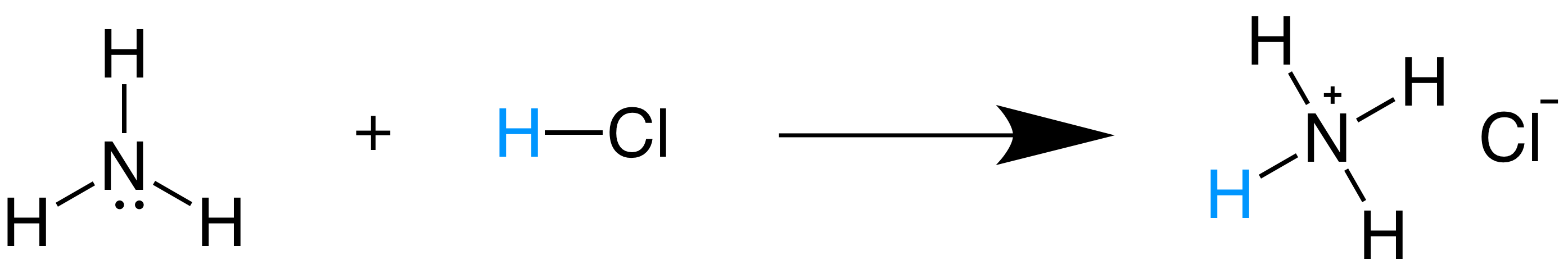SOLVED: What is the conjugate base of HBr? Br+ B Br D: BrOH QUESTION 6 Which compound in the following pair is the stronger acid? CHBCHZCH3 (pKa = 50) and CHBCHZOH (pKa =

PPT - HBr is a strong acid , so the reaction goes to completion. PowerPoint Presentation - ID:4536389
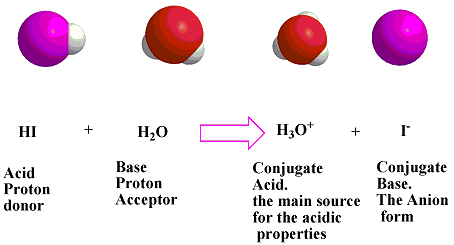
Which pair is a Brønsted–Lowry conjugate acid–base pair? NH_3 ; NH_4^+ or H_3O^+ ; OH^- or HCl; HBr or ClO_4^(-); ClO_3^- | Socratic
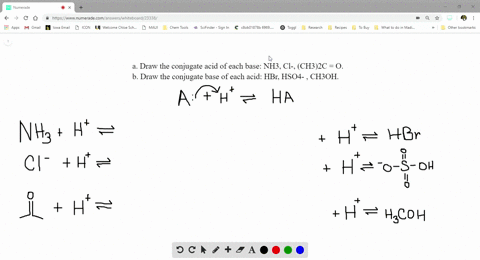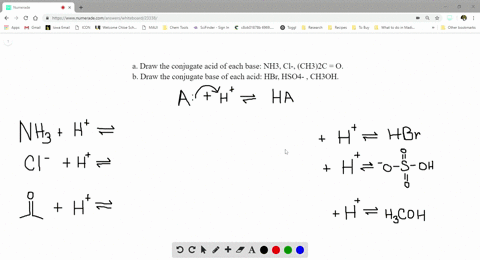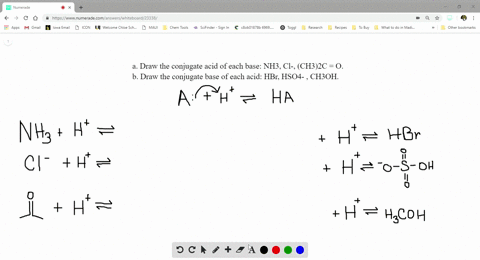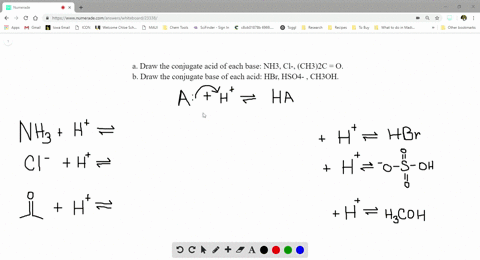
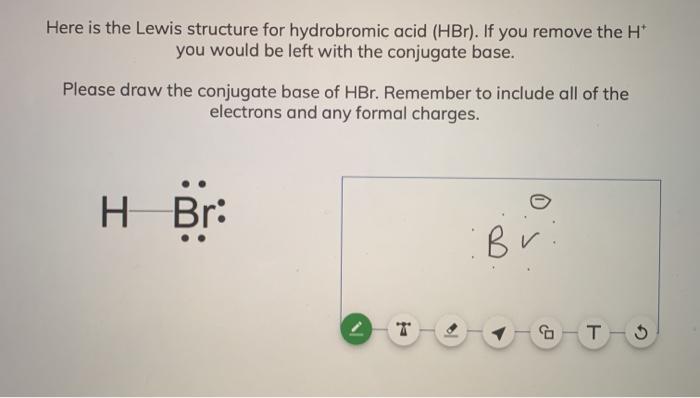
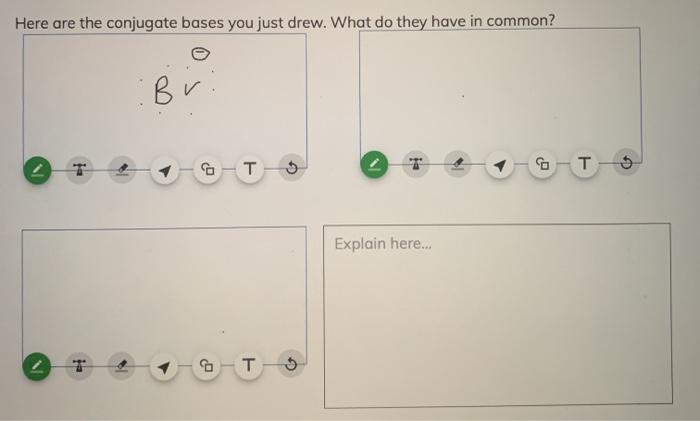
SOLVED:a. Draw the conjugate acid of each base: NH3, Cl^-, (CH3)2C = O. b. Draw the conjugate base of each acid: HBr, HSO4^- , CH3OH.
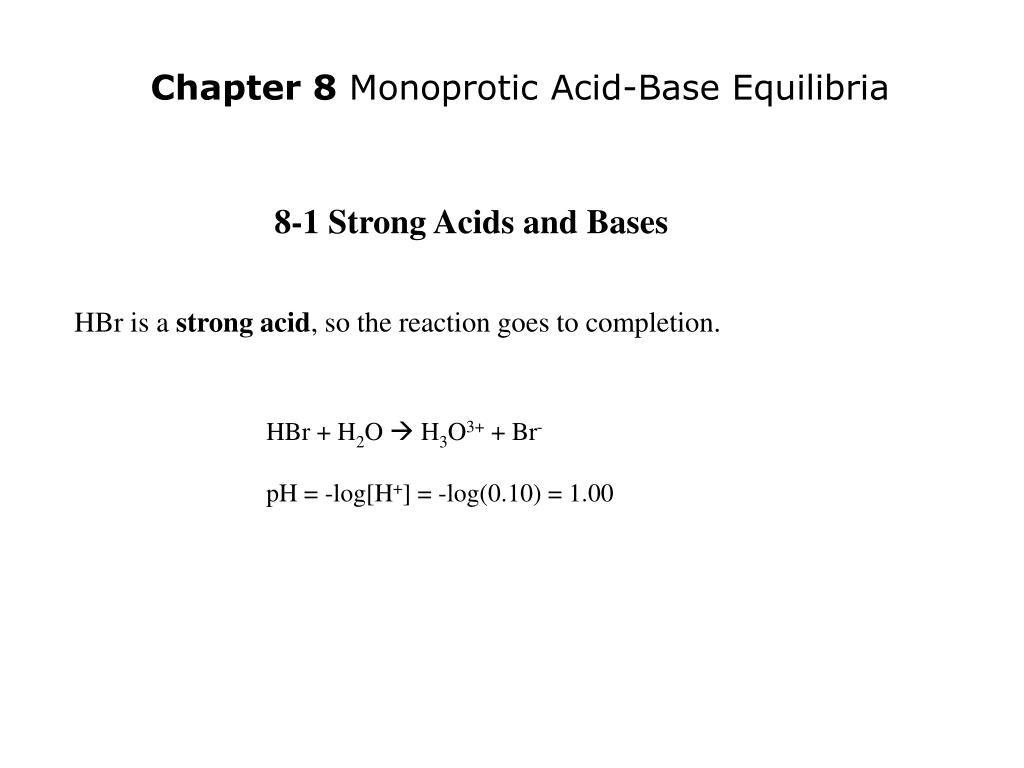
PPT - HBr is a strong acid , so the reaction goes to completion. PowerPoint Presentation - ID:4536389

PHILIPPINE CHEMISTRY PROFESSIONALS BOARD EXAM REVIEWER - Which one of the following mechanistically depicts the acid-base reaction that occurs when hydrobromic acid(HBr) is added to methanol (CH4O)? Please refer to attached image.
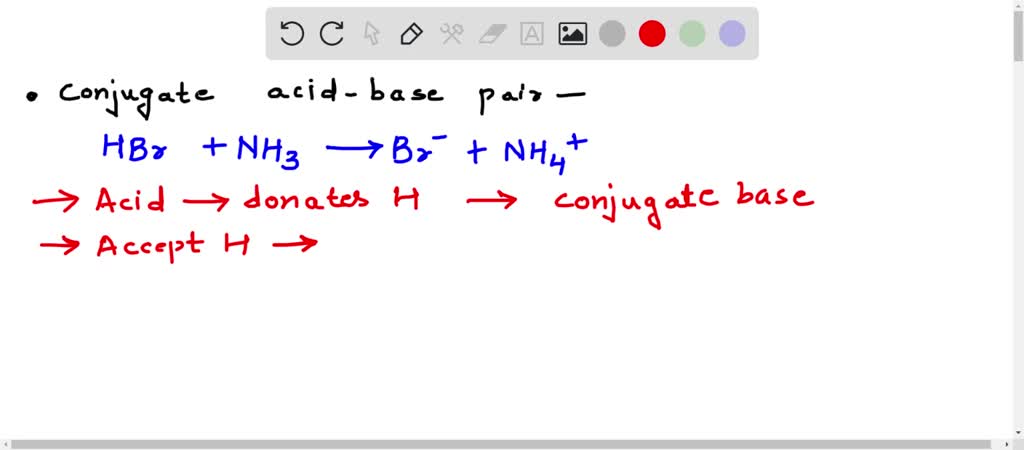
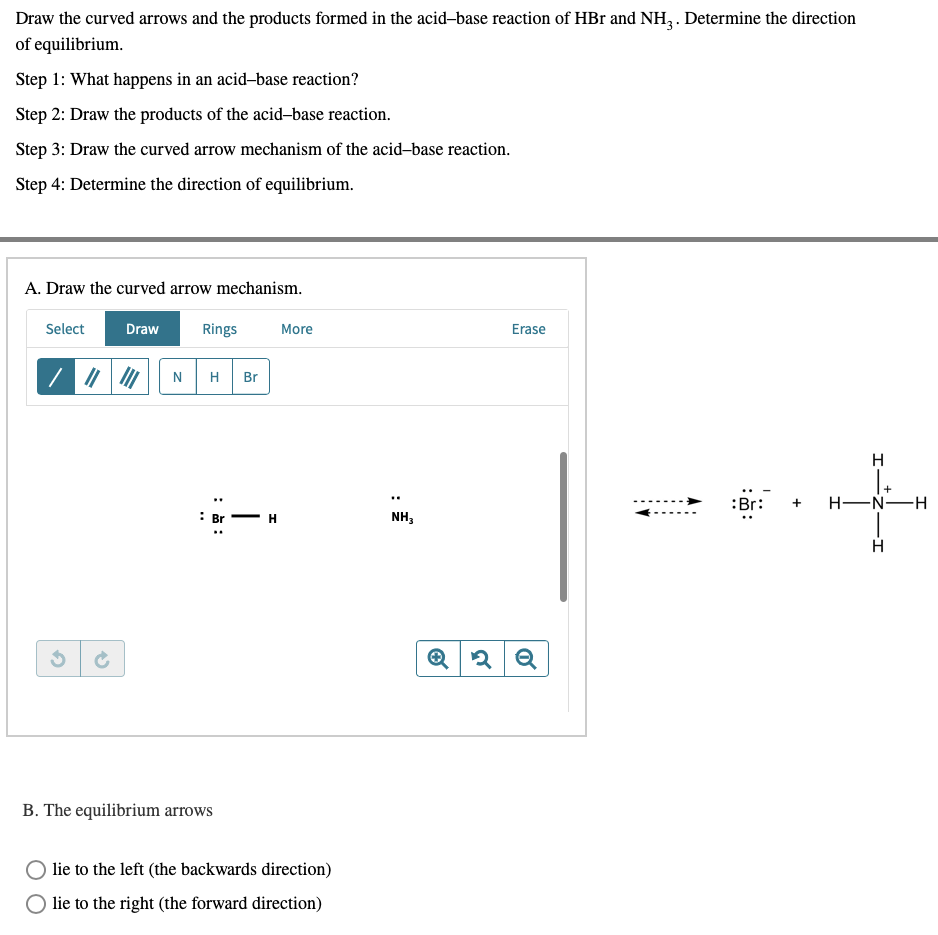
SOLVED: Identify the conjugate acid-base pairs in the reaction shown below HBr + NH3 Br + NH4 acid; chemPad Help Gianke conjugate base: chemPad Help Girekt base: chemPad Help Creke conjugate acid:
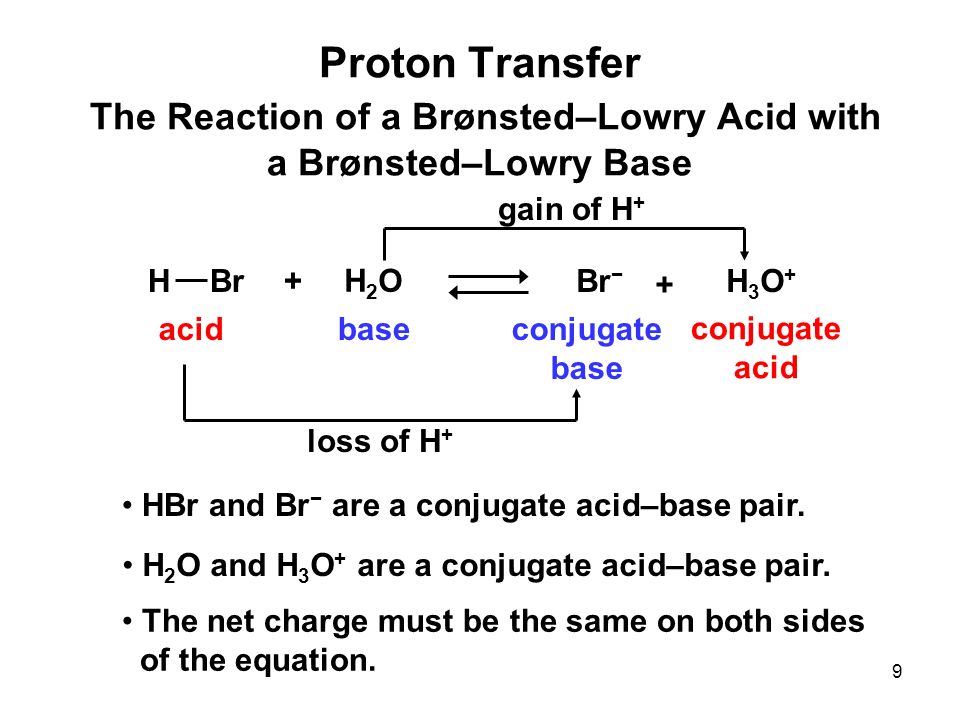
1 Introduction to Acids and Bases The earliest definition was given by Arrhenius: An acid contains a hydrogen atom and dissolves in water to form a hydrogen. - ppt download
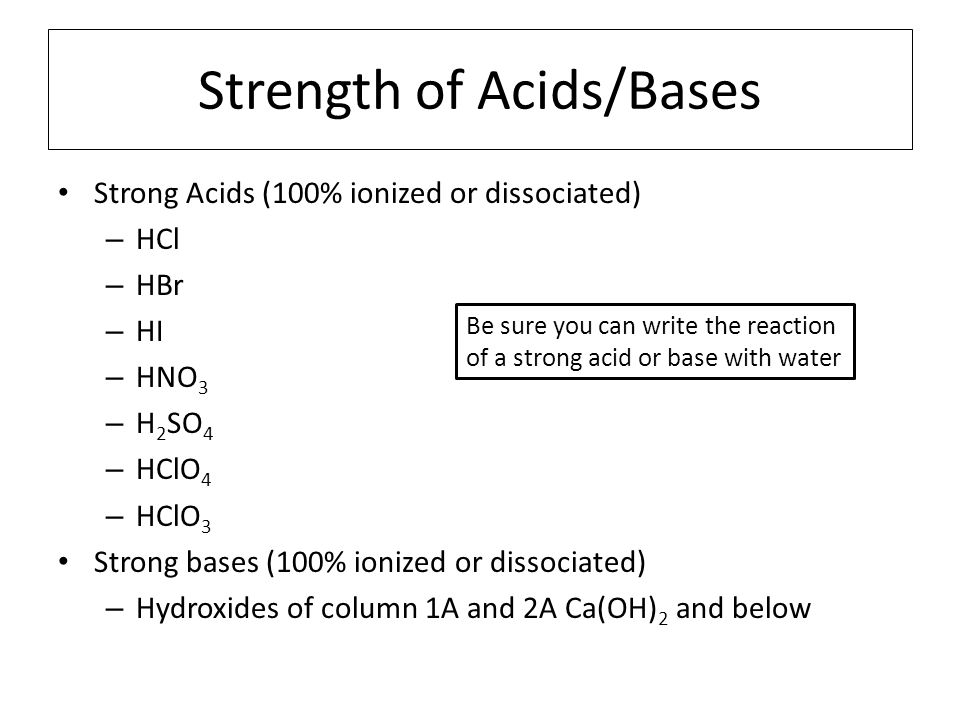
HL Acids and Bases. Strength of Acids/Bases Strong Acids (100% ionized or dissociated) – HCl – HBr – HI – HNO 3 – H 2 SO 4 – HClO 4 – HClO 3 Strong bases. - ppt download