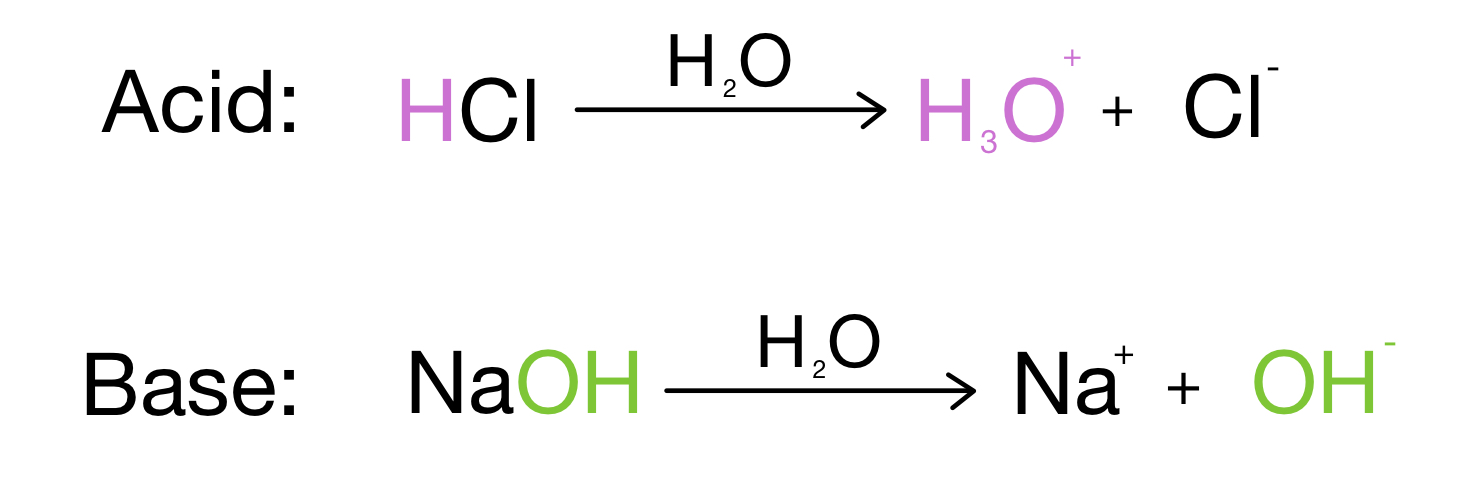
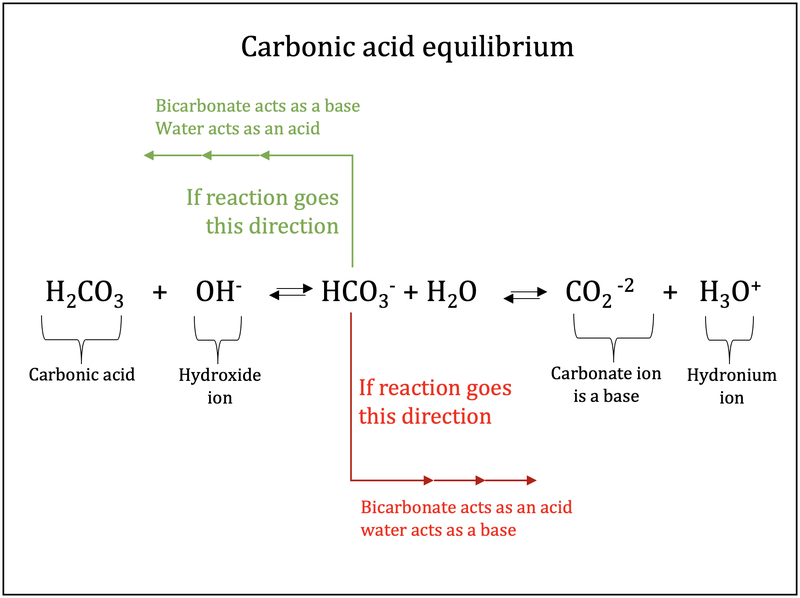
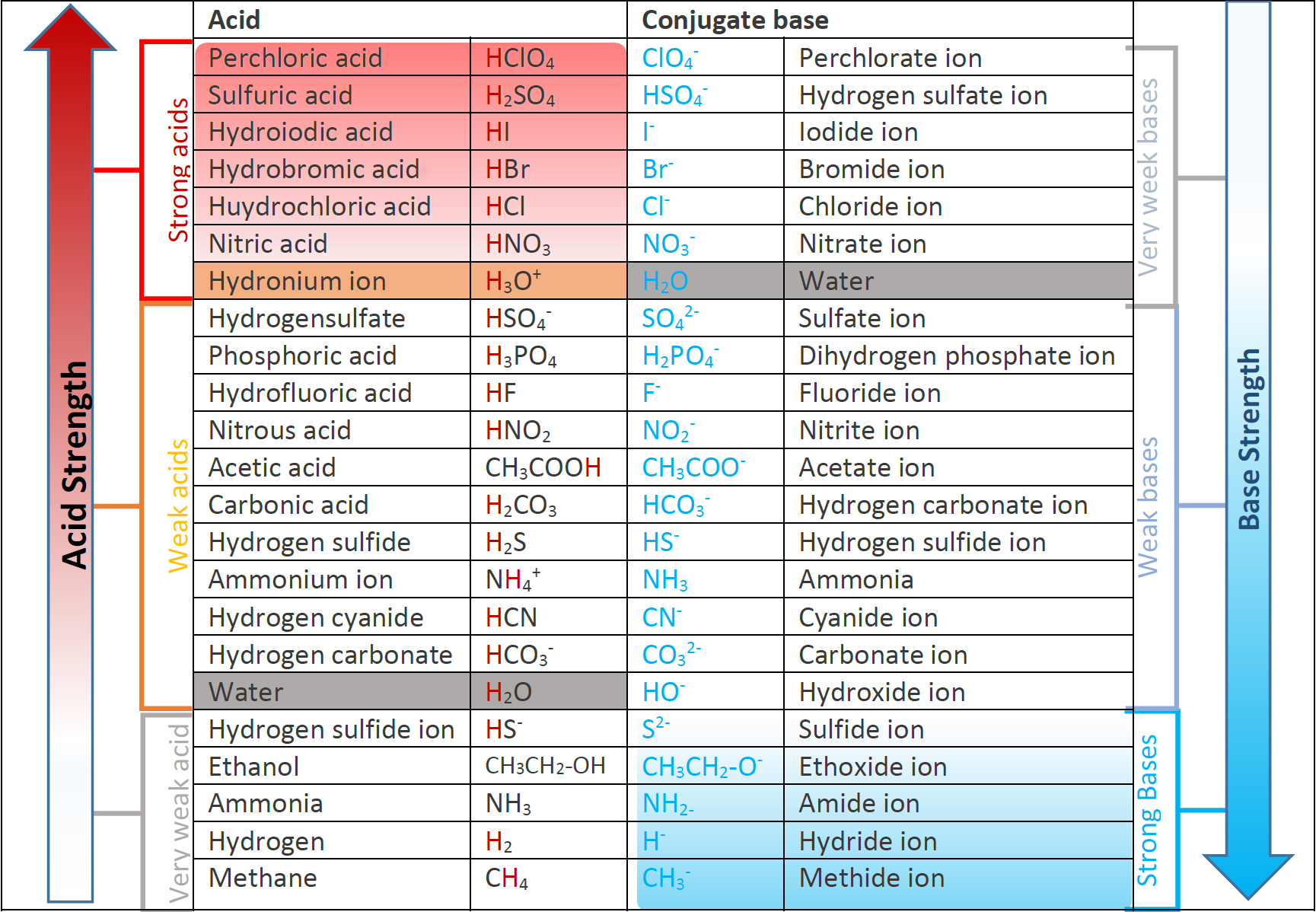
Identify the conjugate acid-base pairs for the reaction (with the acid written first). CN- + H2O = HCN + OH- |CN- / HCN |HCN / CN- |OH- / H2O |H2O / OH- | Homework.Study.com

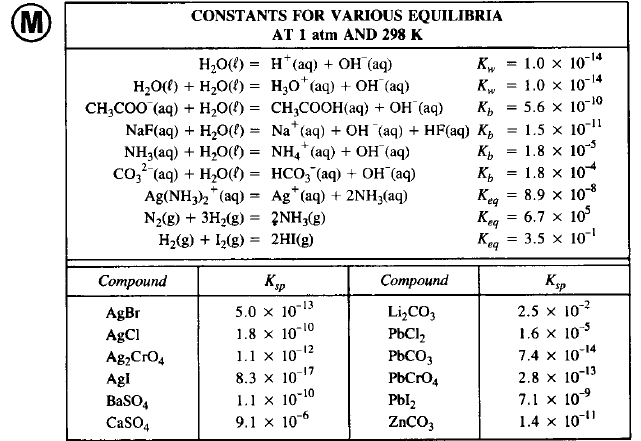
SOLVED: Activity : Acids and Bases Name Date FIII in the missing iniormation Ihe table belox . [H,o | POH [OH | ACID BASE? 11.68 473 % 10 1224 8 53 *






![Calculating pH, pOH, [H+], [OH-] - Acids and Bases Calculating pH, pOH, [H+], [OH-] - Acids and Bases](http://iloveacid--basechemistry.weebly.com/uploads/2/7/8/0/27808151/2222607_orig.gif)